Table Width Extenders
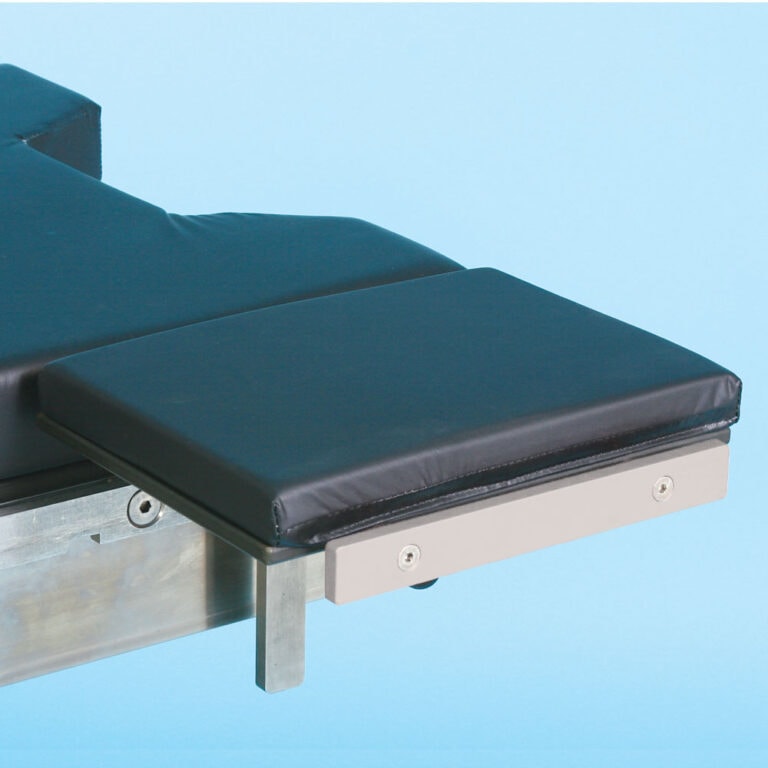
Table Width Extender 11″ X 8″ (28 cm x 20 cm)
(800-0081)
The Table Width Extension, 11 x 8, mounts on the O.R. table side rail offering the surgeon firm support over the surgical table during upper extremity and thoracic surgical procedures. Unique design allows any table accessory to be mounted to this extension. Deluxe pad included. 11″L x 8″W (28 cm x 20 cm).
- Attaches to any O.R. side rail with mounting clamp (sold separately)
- Choose from three available sizes
- Raise and lower height of extension to be level with O.R. table; either with or without x-ray top
- Required Simple Clamp sold separately
Required Accessory
#800-0228 Simple Clamp
Replacement Pad
#508-0132 Table Width Extension Pad, 11 x 8



Table Width Extender 15″ x 8″ (38 cm x 20 cm)
(800-0079)
The Table Width Extension, 15 x 8, mounts on the O.R. table side rail offering the surgeon firm support over the surgical table during upper extremity and thoracic surgical procedures. Unique design allows any table accessory to be mounted to this extension. Deluxe pad included. 15″L x 8″W (38 cm x 20 cm).
- Attaches to any O.R. side rail with mounting clamp (sold separately)
- Choose from three available sizes
- Raise and lower height of extension to be level with O.R. table; either with or without x-ray top
- Required Simple Clamp sold separately
Required Accessory
#800-0228 Simple Clamp
Replacement Pad
#508-0114 Table Width Extension Pad, 15 x 8



Table Width Extender 20″ x 4″ (51 cm x 10 cm)
(800-0077)
The Table Width Extension, 20 x 4, mounts on the O.R. table side rail offering the surgeon firm support over the surgical table during upper extremity and thoracic surgical procedures. Unique design allows any table accessory to be mounted to this extension. Deluxe pad included. 20″L x 4″W (51 cm x 10 cm).
- Attaches to any O.R. side rail with mounting clamp (sold separately)
- Choose from three available sizes
- Raise and lower height of extension to be level with O.R. table; either with or without x-ray top
- Required Simple Clamp sold separately
Required Accessory
#800-0228 Simple Clamp
Replacement Pad
#508-0075 Table Width Extension Pad, 20 x 4



