Surgical Beach Chairs
Powered Surgical Beach Chair
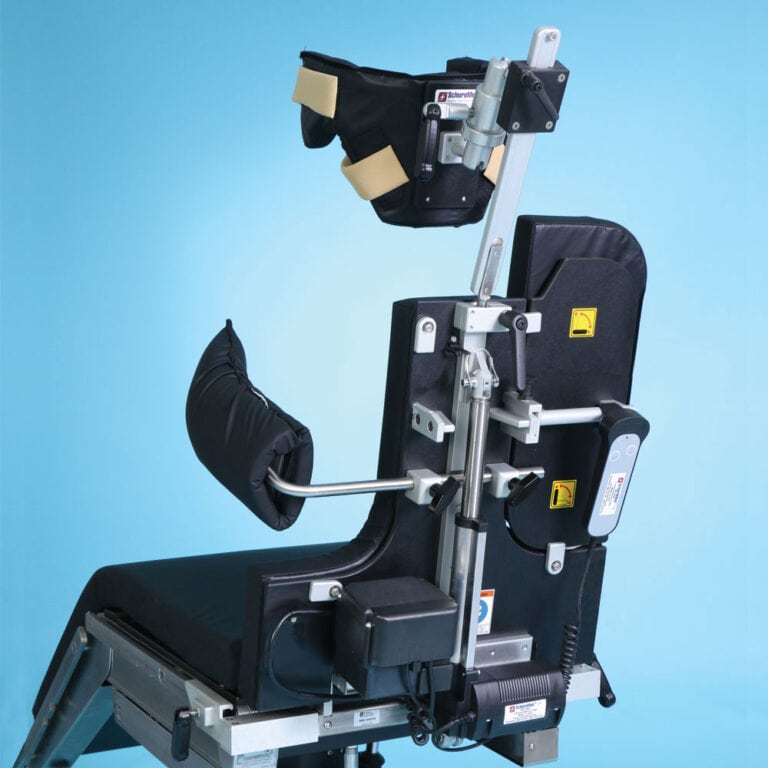
The Powered Surgical Beach Chair by SchureMed delivers a precision-engineered, self-powered positioning solution unlike any other on the market. Featuring an integrated motor system, it operates independently of the surgical table—eliminating reliance on table power sources and offering seamless integration into the most advanced OR environments.
Operative positioning from 0° to 90° is executed in just eight seconds, via ultra-smooth, controlled actuation, affording exceptional procedural accuracy and reducing disruption during lateral repositioning. The system supports an astounding lift of 1,350 lb (612 kg), reliably bearing patients up to 500 lb (227 kg) with assured mechanical robustness.
A hallmark feature is the double-ball-joint headrest, enabling 30° of tilt in every plane—forward, backward, laterally—ensuring secure and adaptable craniocervical support, even in patients with kyphotic curvature. The sliding headrest and five-piece deluxe pad set, along with the included lateral brace and shoulder pull-away panels, combine to maximize access, comfort, and surgical exposure. Attachable to virtually any OR table with integrated adjustable clamps, the device ensures stability while preserving surgical field integrity.
This advanced solution is designed to elevate both surgeon workflow and patient safety. It embodies SchureMed’s commitment to high-precision, high-technology surgical positioning systems.
E-Z Lift Surgical Beach Chair
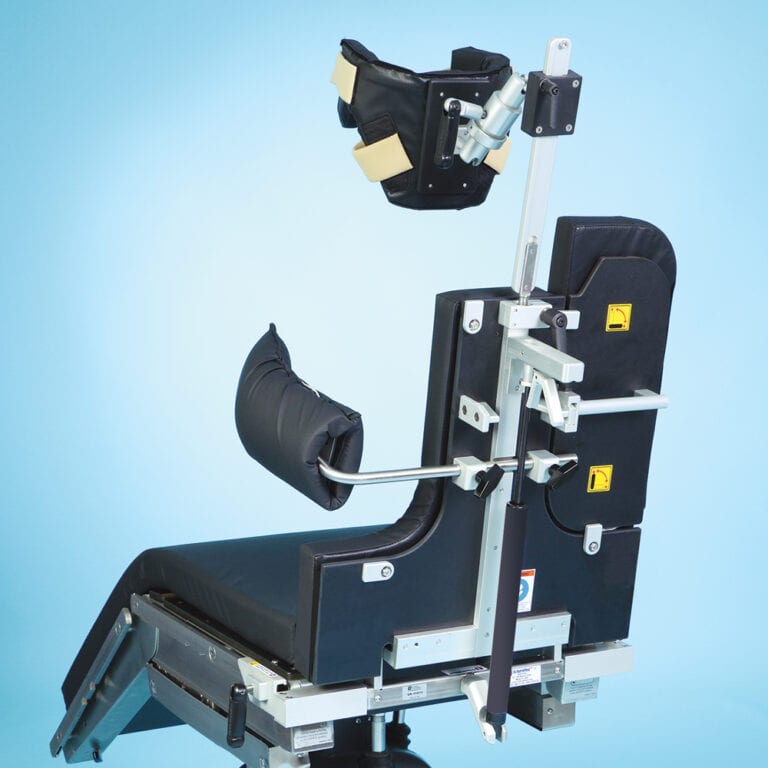
The E-Z Lift Surgical Beach Chair optimizes OR staff ergonomics while preserving the high-tech integrity of surgical positioning. Powered by a robust piston system, it assists staff in transitioning patients from supine to seated positions, achieving 0° to 90° articulation with efficiency and reduced manual effort.
Though mechanically simpler than its motorized counterpart, it retains the same double-ball-joint head system to provide adaptable 30° tilt in all directions, delivering secure cranial support across patient morphologies—including kyphotic anatomy. The lateral brace and deluxe five-piece pad set are inclusive, ensuring both positional stability and enhanced patient comfort.
Supporting up to 500 lb (227 kg), this unit demonstrates that low-effort manual assistance can deliver reliability and safety without compromising procedural sophistication. Crafted as a cost-effective alternative to the Powered Beach Chair, the E-Z Lift model stands out as an ideal choice for operating rooms seeking high-quality performance with streamlined, user-assisted control.
Complete SchureLoc XPS Shoulder System
The Complete SchureLoc XPS Shoulder System embodies SchureMed’s vision of configurable, integrated OR solutions for optimal operative support. This comprehensive kit comprises:
- The SchureLoc™ XPS unit itself, enhancing stability and adaptability.
- A choice of either the Powered Beach Chair or the E-Z Lift Beach Chair, allowing facilities to tailor the system according to automation preferences.
- A Multi-Axis Arm Positioner for fine-tuned limb control.
- A Shoulder Chair Dolly for ergonomic transport and storage.
- The Schure Socket XL, and a Disposable SchureLoc™ XPS Sterile Kit to facilitate sterile environments.
- Disposable Head & Chin Straps for secure patient fixation, ensuring airway preservation.
By integrating advanced positioning mechanics with essential accessories, this system streamlines OR workflows. It enables high-precision shoulder surgery, consolidates equipment into a modular, mobile system, and is tailored to the high-demand nature of modern surgical environments.