Wrist Tower
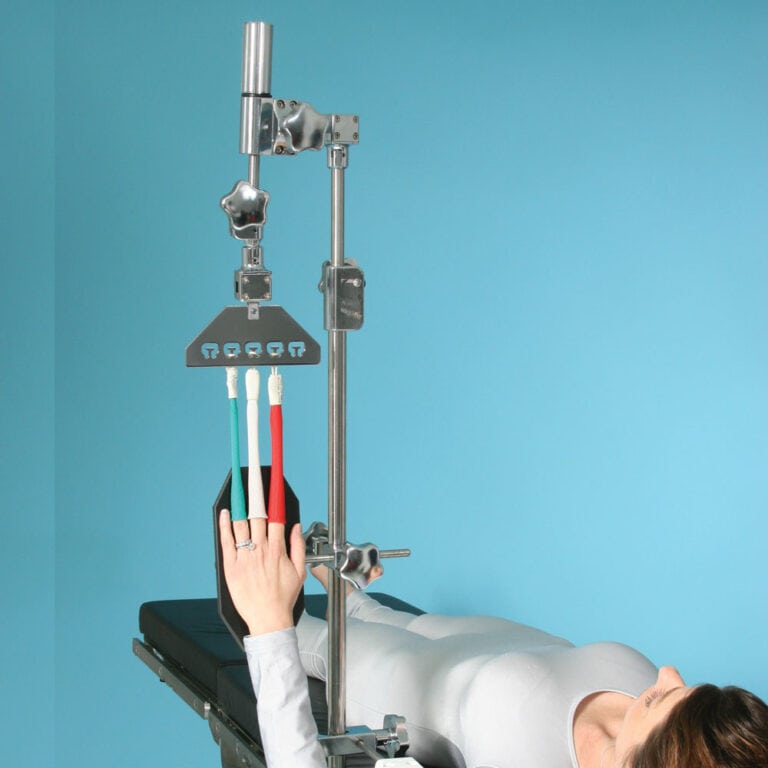
Wrist Tower
(800-0135)
The wrist traction configuration offers a variety of positioning capabilities. You can apply traction by simply dialing the knob from 0-25 lbs (0-11 kg). Pronation and Supination can be easily controlled by the surgeon’s preference and locked in place.
- Simply dial in your tension…eliminates use of sand bags
- All components are autoclavable
- Easily attaches to side rail of surgical table with CamLoc Clamp
- Improves productivity… eliminates the need for assistant to hold extremity
- Required CamLoc Clamp sold separately
Required Accessory
#800-0271 CamLoc Clamp
Limited Use Finger Traps:
#512-0025 Small Single
#512-0024 Medium Single
#512-0023 Large Single
#512-0022 Exrta Large Single
#512-0026 Pediatric Single
#512-0027 XL Double
#512-0028 L Double
#512-0029 M Double
#512-0030 S Double
#512-0031 Pediatric Double

