SchureMed TKR for Total Knee Replacement

SchureMed TKR
(24″ – 800-0141, 30″ – 800-0171)
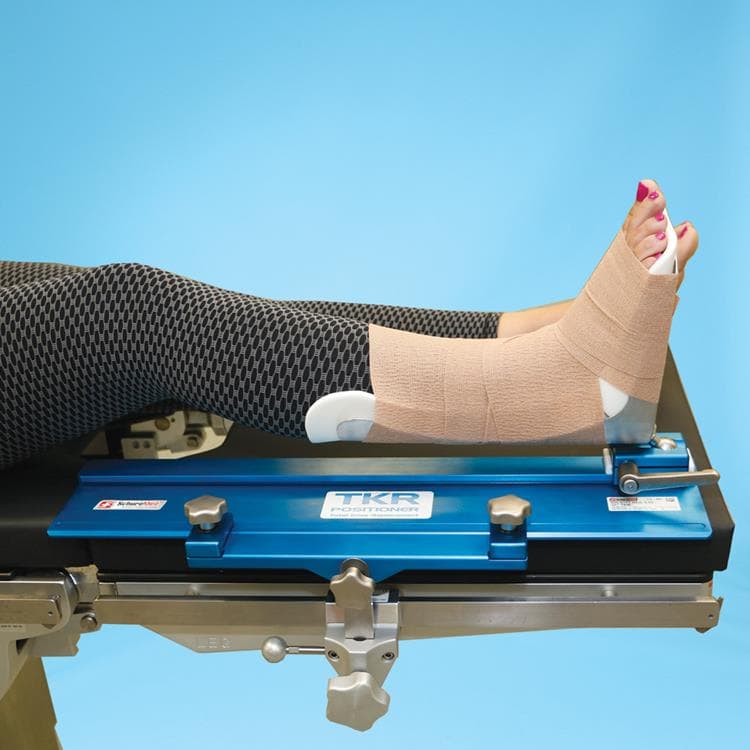
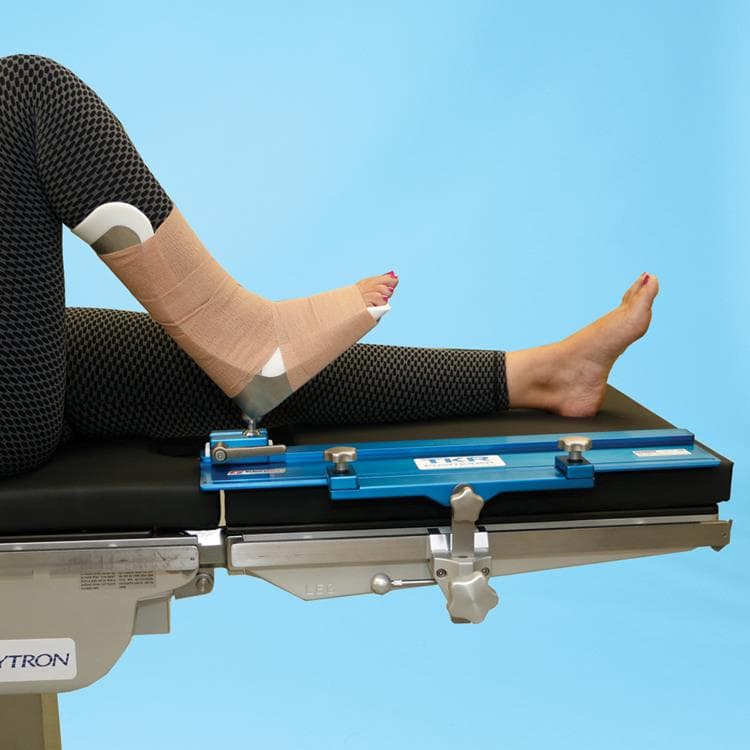
This ball-track system for total knee replacements is a huge leap forward for the surgeon and patient alike. Ball joint at the heel of the leg holder places the operative leg in extension, flexion, tilt and rotation. Easy-to-use and completely rigid once locked. Eliminates the need for additional leg positioning devices, sandbags and gel-pads.
- NEW Custom Sterilization Tray included with purchase
- Track Locking Bracket securely locks the Base Plate to the table with two thumb screws
- Locks in any position with a simple turn of the wrist
- Smooth sliding motion for effortless leg positioning
- Well leg lies flat – eliminating the need for well-leg positioners
- All components are autoclavable
- Required CamLoc Rail Clamp sold separately
- Available in 24″ (61 cm) & 30″ (76 cm)
- Includes (1) TKR Boot Liner
Required Accessory
#800-0271 CamLoc Rail Clamp
Included Accessories
#800-0141-TRAY 24” Sterilization Tray
#800-0171-TRAY 30” Sterilization Tray
Optional Accessory
#800-0146 TKR Boot Liners



