Schure Foot
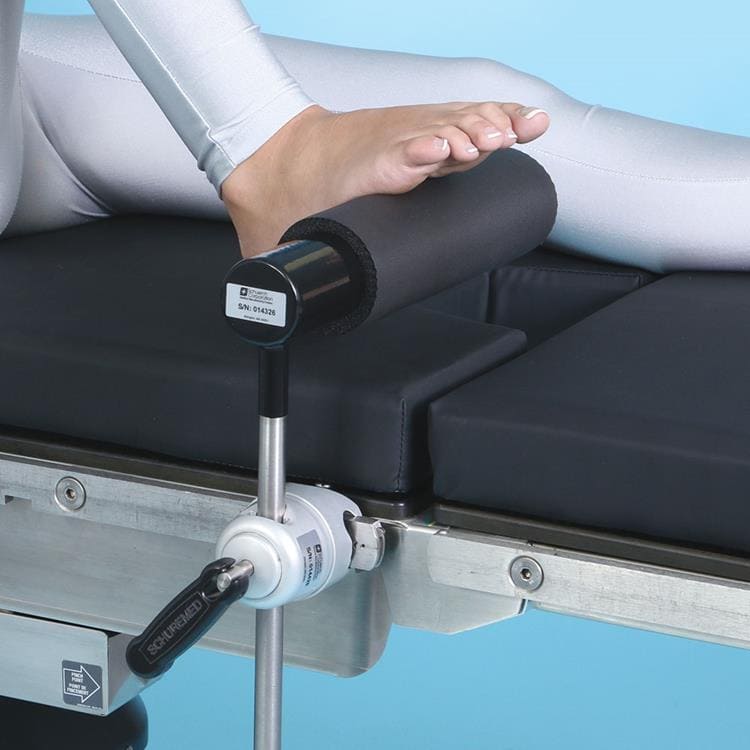
SchureFoot, Stainless Steel
(800-0062)
The SchureFoot for Total Knee Replacement offers a high-quality leg holder to secure the foot during knee surgeries. It easily mounts to operating room side rails using the Schure Socket XL, letting the surgeon replace the entire knee while the patient’s foot is held firmly in place.
- Disposable and conductive foot pad included
- Not made with natural rubber latex
- Now available in radiolucent, clear Lexan as well
- Patient’s well-leg lies flat
- Required Schure Socket XL sold separately
- 12”L x 18″H x 2”D (31 cm x 46 cm x 5 cm) Post
Required Accessory
#800-0134 Schure Socket XL
Replacement Pads
#508-0111 SchureFoot Disposable Pads



SchureFoot, Lexan
(800-0011)
The SchureFoot for Total Knee Replacement offers the same secure positioning as the stainless steel model without the concerns of radiopacity. This radiolucent, clear Lexan version easily mounts to operating room side rails using the Schure Socket XL, letting the surgeon replace the entire knee while the patient’s foot is held firmly in place.
- Disposable, conductive, foot pad included
- Not made with natural rubber latex
- Patient’s well-leg lies flat
- 12”L x 16″H x 2”D (31 cm x 41 cm x 5 cm)
Required Accessory
#800-0134 Schure Socket XL
Replacement Pads
#508-0111 SchureFoot Disposable Pads



