Pediatric Lithotomy Stirrups
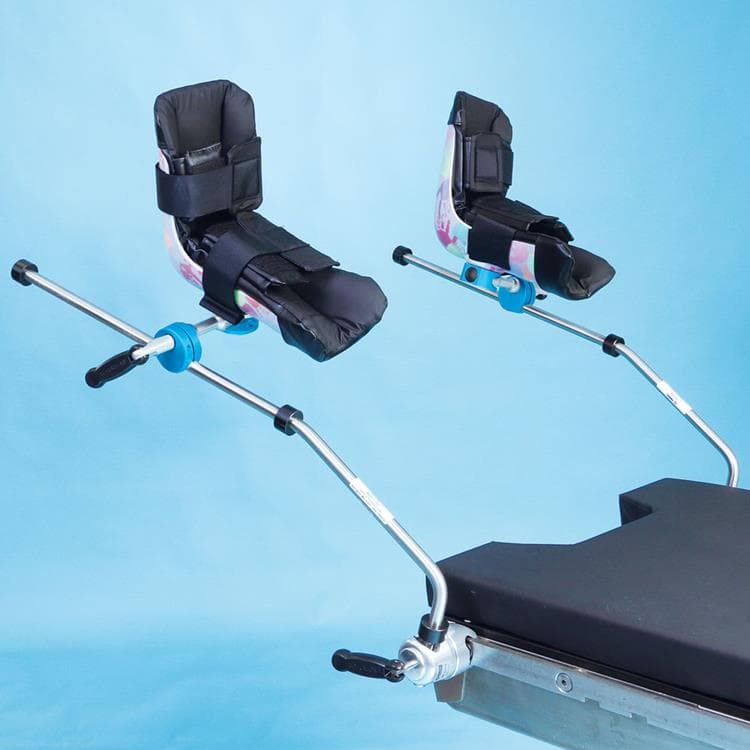
Pediatric Lithotomy Stirrups
(800-0233)
(800-0234)
The Pediatric Lithotomy Stirrups are a comfortable boot stirrup specifically designed with the pediatric patient in mind. Pediatric stirrups attach to side rail of any OR table using the Schure Socket clamps (sold separately). Boot pads are included.
- Pediatric boot stirrup design encapsulates patient’s lower leg
- Floating boot automatically adjusts when repositioning the pediatric boot stirrup
Required Accessory: Purchase Two
#800-0006 Schure Socket
Optional Accessory
#800-0074-P Pediatric Stirrup Dolly
#800-0160 Sterile Clear View Leg Drape, 10/cs
Replacement Pads
#508-1295 PUPS Boot Pads
#508-1296 KIDS Boot Pads



