Lithotomy Stirrups
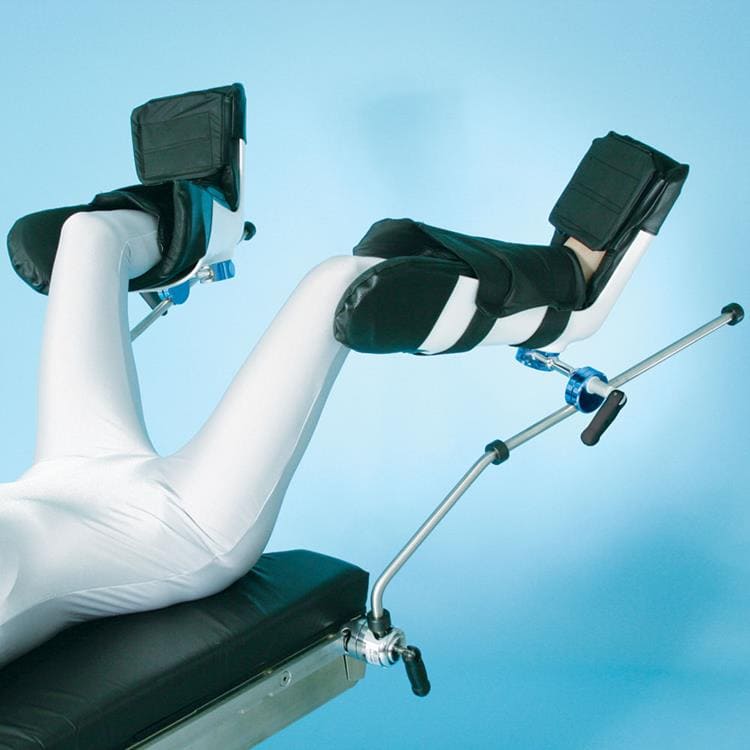
Lithotomy Stirrups
(800-0049)
Patient positioning using the Lithotomy Stirrups features an extended lateral boot design that supports the patient’s leg entirely, preventing superficial nerve damage and prevents unwanted leg rotation intra-operatively. O.R. table side rail clamp sold separately. Sold in pairs.
- Use for long or short procedures
- Extended soft boot pads included
- 350 lb. (159 kg) patient weight capacity
- Two required Schure Socket Clamps sold separately
Required Accessory: Purchase Two
#800-0006 Schure Socket
Optional Accessory
#800-0074-L Lithotomy Stirrups Dolly
#800-0160 Sterile Clear View Leg Drape, 10/cs
Replacement Pad
#508-1415 Platinum Boot Stirrup Pads, Set
Please be advised that you must have an OR table with a side rail on each side that extends from the back section through to the foot section.



