Knee Crutches
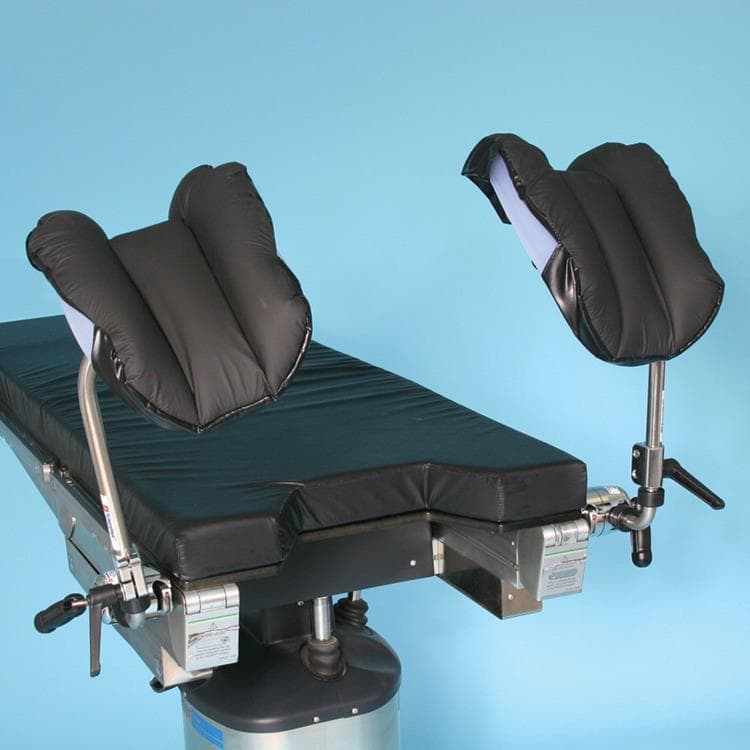
Knee Crutches
(800-0041)
Facilitate a wide-range of patient positions for surgery with this style of leg holders that adjust vertically from 12″ to 19″ (31 cm – 48 cm) and rotate 360º to provide precise patient positioning with a simple, ergonomic, locking handle. Attach to the operating room table with the versatile Schure Socket XL (sold separately). Sold in pairs.
- Constructed of highest-quality stainless steel
- Extremely soft and comfortable crutch liners prevent superficial nerve damage to the patient
- Mount to side rail quickly and securely with Schure Socket XL
- Knee crutch pads included
Required Accessory: Purchase Two
#800-0134 Schure Socket XL
Replacement Pads
#508-0154 Knee Crutch Pads



