Great White HD1000 Stirrups
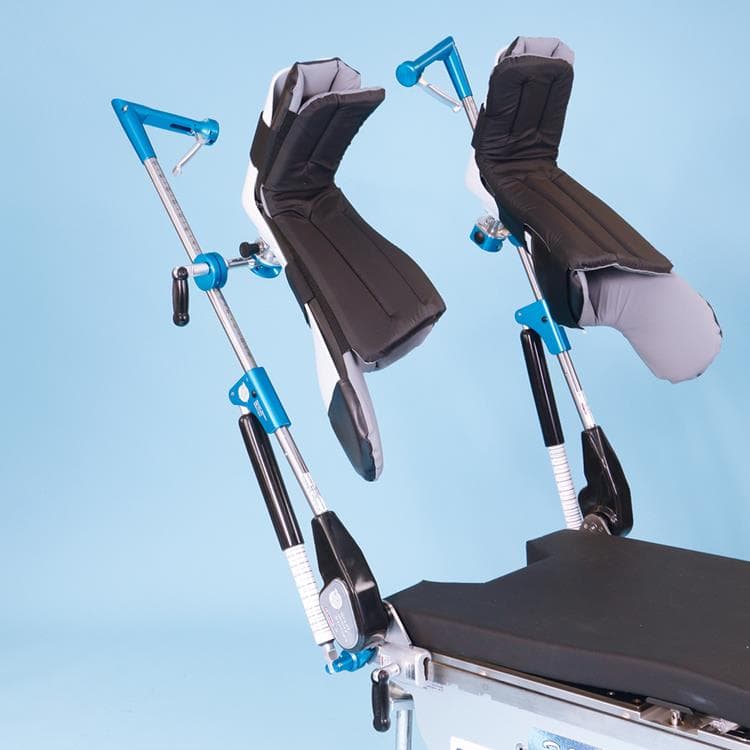
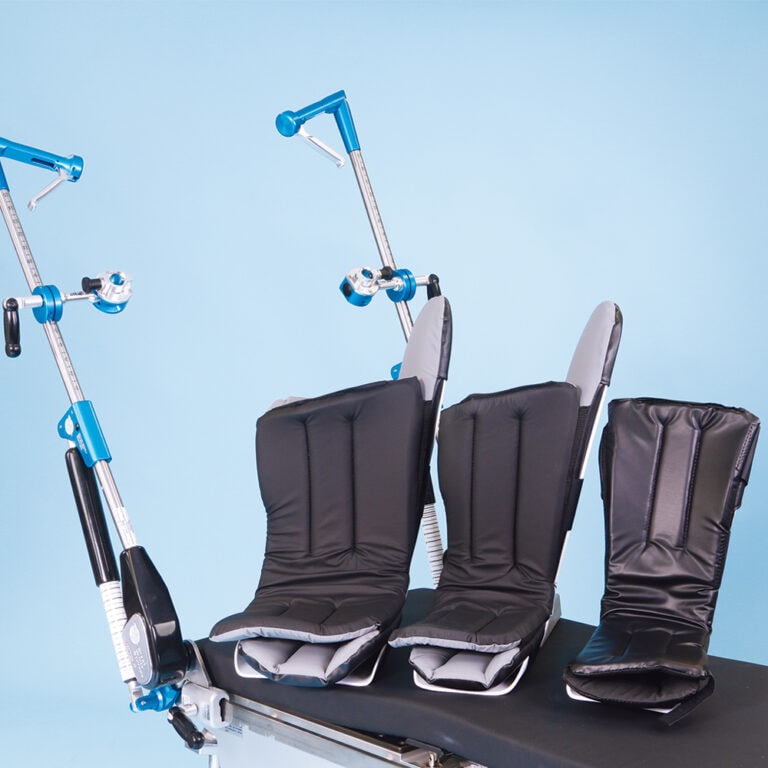
Great White HD1000 Stirrup Boot System
(800-0364)
The Great White HD1000 Stirrups is an interchangeable boot system with 1,000lb (454kg) patient weight capacity.
SchureMed’s stirrup systems provide safe and quick positioning assistance for improved surgical site access.
This intra-operative, adjustable medical stirrup for gynecological, laparoscopic, and urological applications offers exceptionally precise placement, effectively minimizing the possibility of nerve damage. Exclusive axial rotation, high/low lithotomy and abduction/adduction from the handle improves patient positioning.
Required Accessory: Purchase Two
#800-0396 Stirrup Clamps
Optional Accessory:
#800-0160 Sterile Clear View Leg Drape, 10/cs
Replacement Pads:
#508-1415 Platinum Stirrups Boot Pads, Set
#508-1354 Premium Stirrups Boot Pads, Set
#508-1502 Bariatric Stirrup Boot Pads, Set



