Candy Cane Stirrups
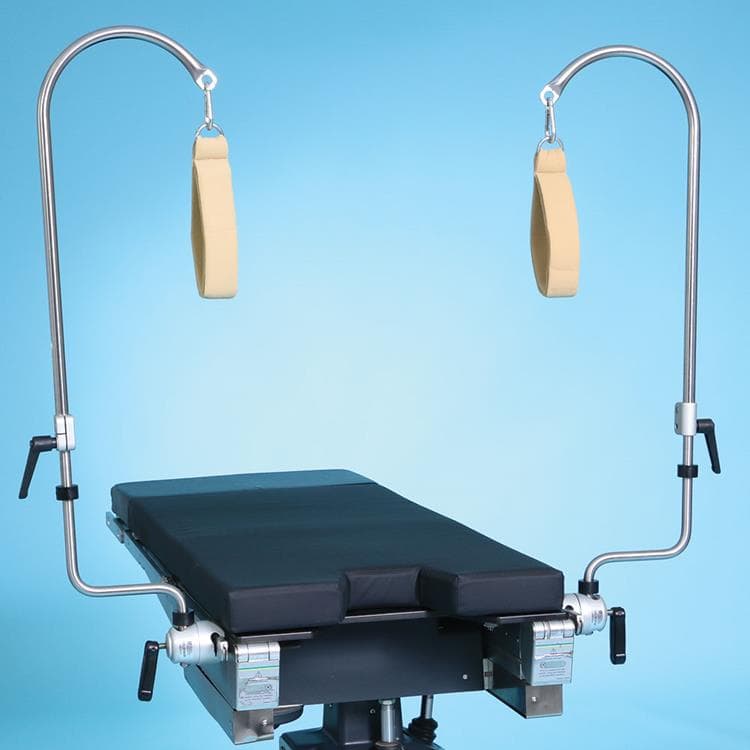
Candy Cane Stirrups
(800-0012)
Candy Cane Stirrups are a cost-effective way to position patients for a wide-range of surgical procedures. Lock the medical stirrup’s height or change the angle of the patient’s leg by simply twisting the ergonomic handles. Candy Cane Stirrup Strap Assembly included.
- Adjust vertically from 28″-44″ (71 cm-112 cm) with an 8″ (20 cm) leg clearance
- 18″L x 1 1/2″W (46 cm x 4 cm) double-looped, cotton ankle strap
- Lock stirrup height by simply twisting the ergonomic handle
- Connect stirrups to OR table side rail with Schure Socket XL (sold separately)
- 350 lb. (159 kg) patient weight capacity
Required Accessory:
#800-0134 Schure Socket XL
Optional Accessory:
#800-0160 Sterile Clear View Leg Drape, 10/cs
Replacement Pads:
#790-0006 Candy Cane Stirrup Strap
#508-0453 Gel Candy Cane Stirrup Sheath



