Lateral Braces
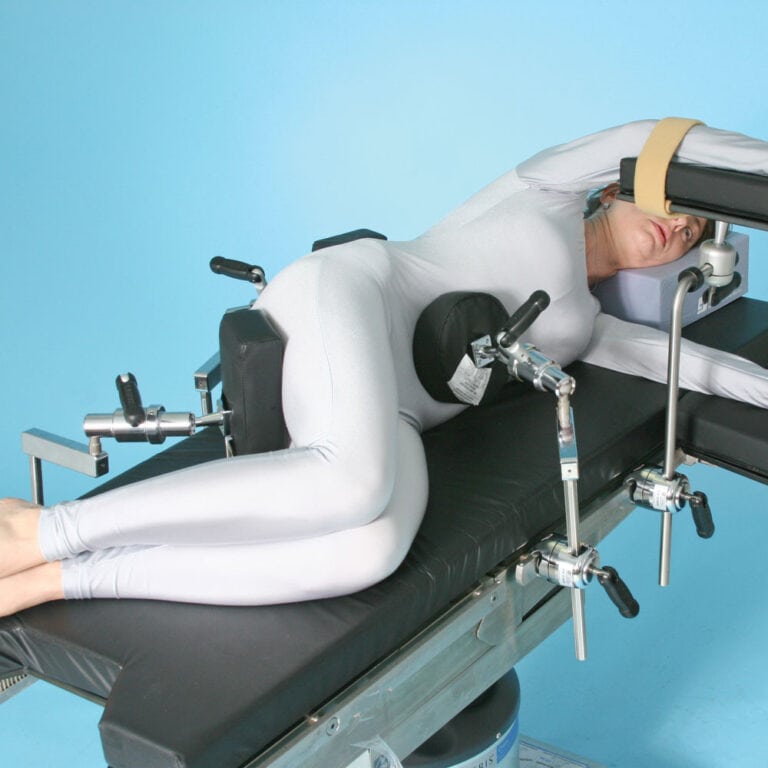
Lateral Braces
(800-0042)
Secure and comfortable positioning for total hip replacement and any other procedure requiring total lateral stability. Three multi-axis braces easily accommodate any size patient for perfect lateral positioning. Pressure relief foam with Deluxe cover provide extreme protection for the patient.
- Simple to set up and easy-to-use
- Clamps quickly to all standard U.S. operating tables
- 5/8″ (1.6 cm) Stainless Steel Mounting Post
- Deluxe foam pads included
- Sold in sets
- 3 required Schure Socket XLs sold separately
- Multi-Axis Arm Postioner (shown) sold separately
Required Accessory: Purchase Three
#800-0134 Schure Socket XL
Optional Accessories
#800-0050 Multi-Axis Arm Positioner
#800-0008 Standard Armboard
#508-0361 Coated Occipital Adult Head Support Pad
Replacement Pads
#508-0116 Rectangle Lateral Brace Pad
#508-0115 Round Lateral Brace Pad

