Great White Robotic Stirrups
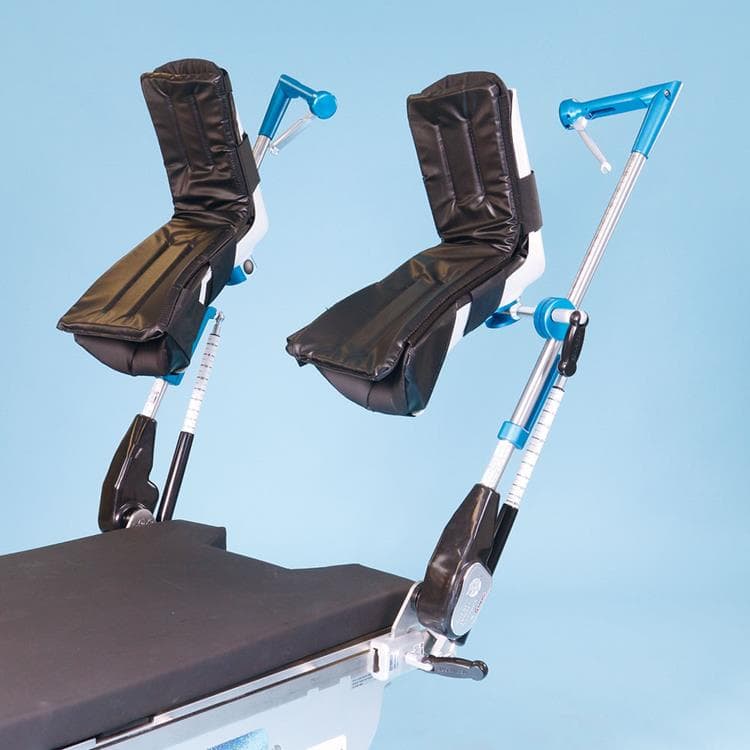
Great White Robotic Stirrups
The mounting blade design quickly and easily inserts into side-rail clamps. The Great White Robotic Stirrups’ range of motion is 55° low lithotomy to 60° high lithotomy. Adduction 9° and abduction 25° and also externally rotates out 20°, with the control to lock the medical stirrup for positioning anywhere in between. Two required Stirrup Clamps sold separately.
- 600 lb (272 kg) patient weight capacity
- Rotates on three axes
- Flexible boot accommodates larger patients
- Lift assist allow easy control with minimal effort
Required Accessory: Purchase Two
#800-0396 Stirrup Clamps
Optional Accessories
#800-0074-R Stirrup Dolly
#800-0284 Wall Rack
#800-0342-RF (with fins)
#800-0342-R (without fins)
Replacement Pads
#508-1354 Premium Stirrup Boot Pads, Set
#508-1415 Platinum Stirrup Boot Pads, Set

